Samsung Biologics to develop Kanaph Therapeutics' retinal disease treatment
By Lim Jeong-yeoPublished : Sept. 24, 2020 - 15:22
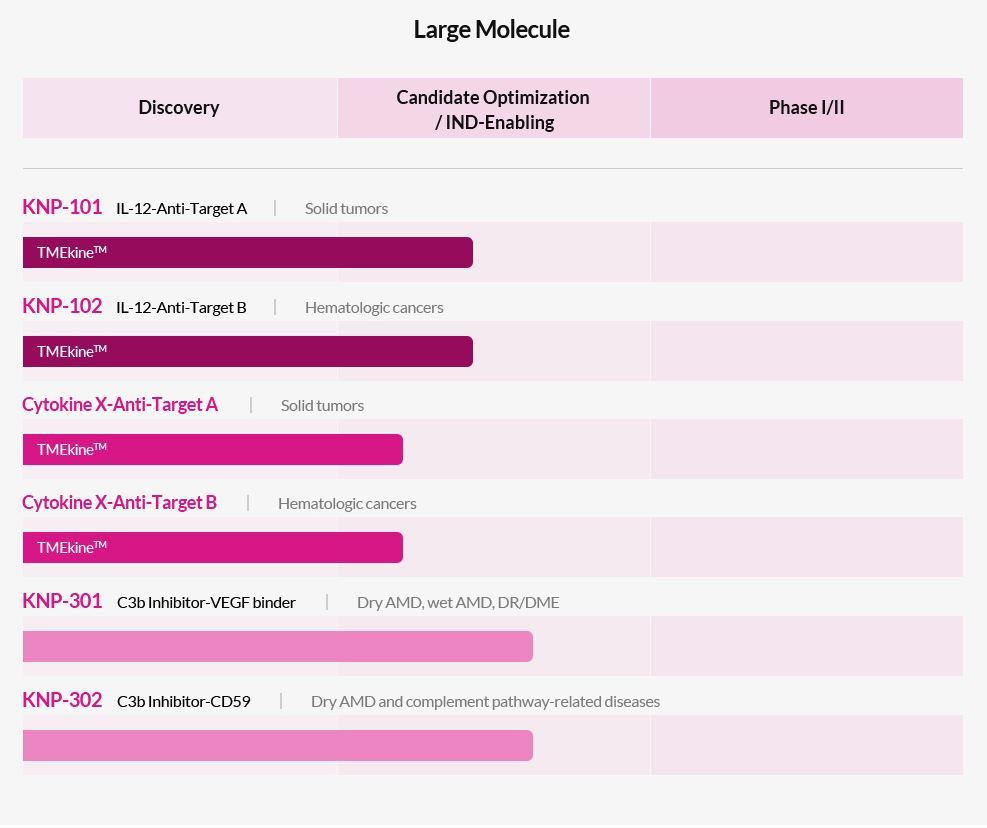
Samsung Biologics will contract develop Kanaph Therapeutics’ retinal disease treatment, the company announced Thursday.
Kanaph’s KNP-301 is a C3bg Inhibitor-VEGF binder novel drug candidate that treats dry and wet macular degeneration, diabetic retinopathy and diabetic macular edema.
Samsung Biologics will undertake all steps of the drug development, spanning cell line development, development process optimization and preclinical and clinical sample manufacturing.
“For a bioventure to trust its first pipeline development to us reflects the client‘s belief in our CDO (contract development organization) service quality,” Samsung Biologics CEO Kim Tae-han said. “By successfully developing this retinal disease treatment, whose core lies in the processing and lyophilization, we will show our CDO expertise to the world.”
Kanaph has a number of novel drug pipelines that are lined up to enter clinical phase trials.
“By partnering with Samsung Biologics, and through the network of contract research organizations and in-house experts, Kanaph will successfully complete global clinical trials,” said Lee Byoung-chul, CEO of Kanpah Therapeutics.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)
Kanaph’s KNP-301 is a C3bg Inhibitor-VEGF binder novel drug candidate that treats dry and wet macular degeneration, diabetic retinopathy and diabetic macular edema.
Samsung Biologics will undertake all steps of the drug development, spanning cell line development, development process optimization and preclinical and clinical sample manufacturing.
“For a bioventure to trust its first pipeline development to us reflects the client‘s belief in our CDO (contract development organization) service quality,” Samsung Biologics CEO Kim Tae-han said. “By successfully developing this retinal disease treatment, whose core lies in the processing and lyophilization, we will show our CDO expertise to the world.”
Kanaph has a number of novel drug pipelines that are lined up to enter clinical phase trials.
“By partnering with Samsung Biologics, and through the network of contract research organizations and in-house experts, Kanaph will successfully complete global clinical trials,” said Lee Byoung-chul, CEO of Kanpah Therapeutics.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)








![[Graphic News] More Koreans say they plan long-distance trips this year](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050828_0.gif&u=)
![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)






![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)

