Korea recalls 59 more hypertension drugs for potential cancer risks
By Sohn Ji-youngPublished : Aug. 6, 2018 - 15:30
South Korea’s Ministry of Food and Drug Safety said Monday that it ordered a recall of 59 hypertension drugs containing valsartan produced by a local drug substance manufacturer, due to its inclusion of above-permitted levels of a chemical that could cause cancer.
After an inspection of all valsartan locally produced or imported to Korea, the drug regulator found that some batches of valsartan produced by Daebong LS contained more than 0.3 ppm of N-nitrosodimethylamine, or NDMA, a chemical that could increase the risk of cancer.
Daebong LS has been importing ingredients for its valsartan product from China’s Zhuhai Rundu Pharmaceutical, according to the ministry.
After an inspection of all valsartan locally produced or imported to Korea, the drug regulator found that some batches of valsartan produced by Daebong LS contained more than 0.3 ppm of N-nitrosodimethylamine, or NDMA, a chemical that could increase the risk of cancer.
Daebong LS has been importing ingredients for its valsartan product from China’s Zhuhai Rundu Pharmaceutical, according to the ministry.

As a result, the Korean drug agency has ordered 59 hypertension tablets sold by 22 pharma companies, containing valsartan sourced in from Daebong LS, to be banned from further sales and prescription.
Among the recalled drugs are Pfizer’s Norvasc V tablets marketed locally by LG Chem, as well as valsartan-containing tablets by Anguk Newpharm, JW Pharmaceutical, Daehwa Pharmaceutical, Huons and Dongwha Pharmaceutical.
The latest recall raises concerns over the safety of hypertension drugs sold here after alarming revelations that 115 locally sold drugs containing valsartan sourced from a Chinese company had contained problematic levels of cancer-inducing chemicals.
Last month, the Korean drug regulator ordered a total of 115 drugs containing valsartan from 54 pharma companies to terminate sales or production after China’s Zhejiang Huahai Pharmaceutical informed the European Medicines Agency that it had identified NDMA in its valsartan.
Similar actions have been taken globally, including the US where the Food and Drug Administration has initiated a recall of some valsartan tablets that contained above-permitted levels of valsartan.
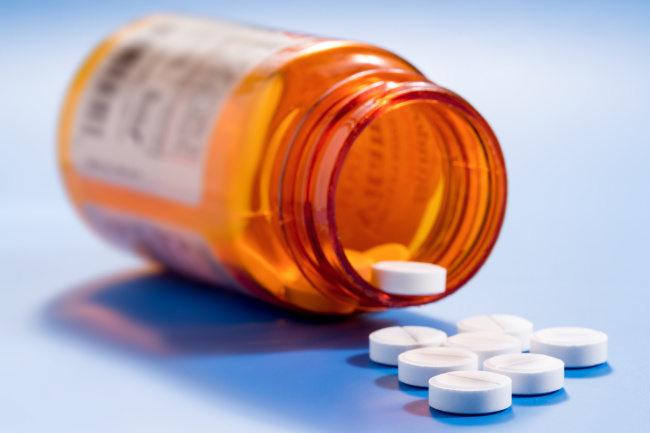
Valsartan is a medication primarily used to treat high blood pressure and heart failure. In Korea, a total of 181,286 patients had been taking the drug according to prescription records as of Monday, the ministry said.
The Korean Ministry estimates that if a person were to take the highest valsartan dose of 320 milligrams from the recalled batches for three years -- the period during which valsartan produced by the Chinese company were sold here -- the chances of contracting cancer would be 1 in 180,000.
Meanwhile, the US FDA has concluded that under the same circumstances over four years, the likelihood of cancer occurrence was 1 in 8,000. The EMA has concluded that over seven years, the chances would come to 1 in 5,000.
By Sohn Ji-young (jys@heraldcorp.com)











![[Today’s K-pop] BTS pop-up event to come to Seoul](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050734_0.jpg&u=)




![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)

![[Today’s K-pop] Zico drops snippet of collaboration with Jennie](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/04/18/20240418050702_0.jpg&u=)